Technical Information
1-3. Cooling principle
It explains the principles of cooling, the laws of heat transfer, and the three states of matter.
To achieve the air conditioning described in "1-2. What is air conditioning?", it is necessary to heat or cool the air.
We heat and cool things in our daily lives, so it feels like we can change temperature very easily, but what is the principle behind this?
We will explain the principles of cooling, which are also related to the mechanisms of air conditioners and chillers.
(1) What does "cool" mean?
There are many tools and devices around us that cool things. For example, if you just look around your home, you will see fans, electric fans, room air conditioners, refrigerators, and so on.
Although these tools and devices have different mechanisms, they all share the same fundamental principle of cooling things.
When you fan yourself in a hot room, the wind you create removes the heat around your body, and the evaporation of sweat removes heat from your skin, making you feel cooler.
From this, we can understand that cooling means removing heat.
You can also see that when liquid (sweat in this example) evaporates, heat is also removed (latent heat of vaporization, which will be explained later). The practice of sprinkling water on the ground at dusk in the summer also makes use of this late heat of vaporization.
Fans and electric fans are intuitive and easy to understand, but room air conditioners and refrigerators also work in principle in the same way.
Room air conditioners and refrigerators use the properties of refrigerant to create environment with a temperature significantly lower than the ambient temperature.
(2) Heat transfer (heat exchange)
When two objects of different temperatures come into contact, heat transfers from the "hot" object to the "cold" object. When this happens, from the perspective of the "hot" object, heat is taken away and it is cooled, and conversely, from the perspective of the "cold" object, heat is received and its temperature rises, in other words, it is heated. This is called heat exchange, and the basic principle is that heat always transfers from hot to cold.
There are three laws regarding the rate and amount of heat exchange:
1. Large contact area
2. Heat transfers more smoothly between materials that conduct heat well.
As the name suggests, materials that conduct heat well (have high thermal conductivity) conduct heat quickly.
For this reason, materials with good thermal conductivity, such as copper and aluminum, are used in the heat exchangers of air conditioners.
On the other hand, materials that do not conduct heat well, such as glass wool, are used as insulation.
3. There is a large temperature difference between the materials being heat exchanged.
The greater the temperature difference between two substances, the greater the heat transfer. As the heat exchange continues, the heat transfer decreases until the temperature difference disappears (equilibrium state).
Based on these laws of heat exchange, air conditioners such as room air conditioners often use fin-and-tube heat exchangers in which cold refrigerant flows through copper tubes (tubing) and is forced to blow air through the gaps between aluminum fins that are tightly attached to the copper tubes to make efficient contact with aluminum fins that have a large surface area.

Fin and Tube Heat Exchanger
In addition to heat exchangers that exchange heat between liquids and gases, such as fin-and-tube heat exchangers, there are also other types of heat exchangers that exchange heat between liquids, such as plate heat exchangers and shell-and-tube heat exchangers.
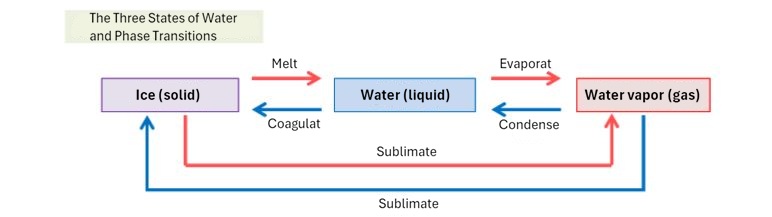
(3) Three states of matter and heat
What happens when you add heat to water? Of course, the temperature rises. However, we know the temperature at which the water's temperature stops rising no matter how much you heat it.
Once the temperature of water reaches 100°C, it will no longer rise any further. Instead, it will boil and evaporate into water vapor. Similarly, when heat is applied to ice, the temperature will not rise above 0°C, and will not rise until the ice has completely melted and turned into water.
The same is true when heat is removed; no temperature change occurs until all the 100°C water vapor has turned into water, and all the 0°C water has turned into ice.
The heat used to change the temperature of water without causing a change in state is called sensible heat, and the heat used to change state without causing a change in temperature is called latent heat.
Comparing the magnitude of the latent heat and sensible heat of water, if the amount of heat required to raise the temperature of water from 0°C to 100°C is set at 100, the latent heat of freezing and melting (state change between ice and water) is 80, and the latent heat of evaporation and condensation (water and steam) is 540.
We can see that the latent heat of evaporation and condensation (water ⇔ steam) is significantly larger than the others. In other words, when a substance evaporates, it takes in a large amount of heat from its surroundings, and when it condenses, it releases a large amount of heat to its surroundings.
freezing cycle used in air conditioning effectively utilizes this principle to cool a room. We will discuss this mechanism in the next section.
Previous item: 1-2.What is air conditioning?
Next item: 2-1. freezing cycle
People who viewed this page also checked out these documents:
Inquiry
For product inquiries, quote requests, etc.
Please feel free to contact us.













