Control Panel Cooling units Technical Information
3-10. Psychrometric chart
In order to understand the enthalpy and specific enthalpy, there is something called a "moist air chart".
A "moist air chart" is a chart that compares and contrasts the interrelationships of dry bulb temperature, relative humidity, absolute humidity, specific enthalpy, etc., which are difficult to grasp for unsaturated air.
Types of moist air chart
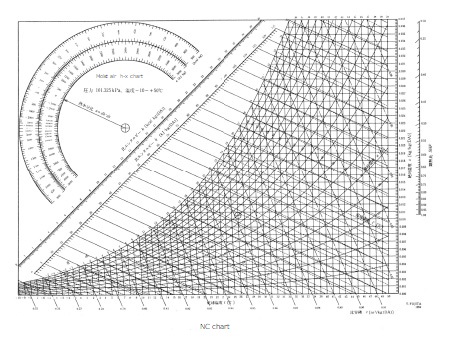
NC chart
The dry bulb temperature will be a moist air chart from -10°C to 50°C.
This is the type most commonly used in general industrial air conditioning, such as air conditioning for people.
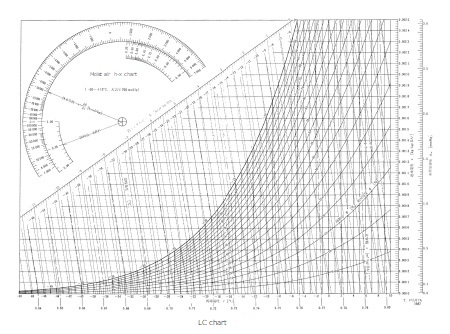
LC chart
This is a moist air chart used when the dry-bulb temperature is low, which cannot be handled by the NC chart.
The dry bulb temperature is -40°C to 10°C and the absolute humidity is 0 to 0.007kg/kg (DA).
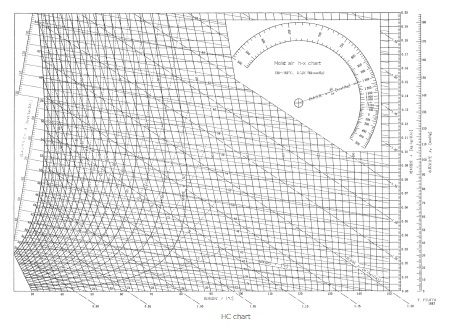
HC chart
This is a moist air chart used when the dry-bulb temperature is high, which cannot be handled by the NC chart.
The dry bulb temperature is 0°C to 120°C and the absolute humidity is 0 to 0.020kg/kg (DA).
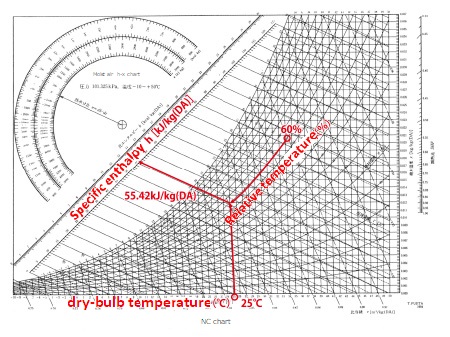
Specific enthalpy on the moist air chart
Example: Specific enthalpy at 25°C, 60%
| Dry bulb temperature | The air temperature as indicated by a dry bulb thermometer |
|---|---|
| Wet bulb temperature | The air temperature as indicated by a wet bulb thermometer. It shows a lower temperature than a dry bulb thermometer due to the latent heat when evaporating water. From the temperature difference at this time, the relative humidity of the air can be determined. |
| Relative temperature | Expressed as water vapor partial pressure/saturated water vapor pressure x 100. The water vapor partial pressure is the pressure of the water vapor contained in the moist air, and the saturated water vapor pressure is the pressure of the vapor when the moist air is saturated. |
| Absolute temperature | Weight of water vapor contained in 1 kg of dry air. The unit is kg/kg (DA). |
| Saturated water vapor | The maximum amount of water vapor (g) that air at a certain temperature can hold in a space of 1m³. If the temperature is low, it will be small, and if the temperature is high, it will be large. |
| Sensible heat | From 0 to 100°C, the temperature of water changes only due to changes in the amount of heat. The amount of heat (energy) required at this time. |
| Latent heat | It is the amount of heat (energy) required to change the form of a substance, such as water ⇔ ice and water ⇔ steam. |
Previous item: 3-9. Enthalpy
Next item: 3-11. Air Conditioning Process and Psychrometric Chart
We're here to give you quick answers to your questions.
