Technical Information
2-1. Freezing cycle
This explains the mechanism of freezing cycle used in air conditioners and cooling devices.
(1) What is freezing cycle?
In "1-3. Principles of Cooling," we learned the principles of cooling things. When a substance evaporates, it absorbs a large amount of heat from its surroundings, and when it condenses, it releases a large amount of heat into its surroundings.
Based on this principle, in order to continue cooling a space, a substance with a large latent heat of vaporization must be continuously supplied to the space to be cooled in a cold liquid state and continue to evaporate.To do this, heat must be absorbed from the evaporated substance, returning it to its original cold liquid state.
The substance that transfers heat is called refrigerant. The system that controls the state and temperature changes of refrigerant to provide continuous cooling is called freezing cycle.
(2) freezing cycle mechanism
The vapor compression freezing cycle is most commonly used in room air conditioners and industrial cooling systems. It consists of four elements: a compressor, a condenser, an expansion valve (capillary), and an evaporator. The inside of the system is sealed, and refrigerant circulates in a certain direction while changing state to provide cooling.
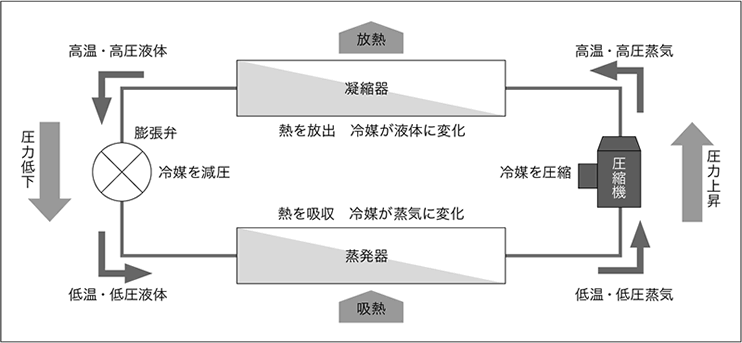
① Compression
The gas refrigerant that has absorbed the heat must be fed back into the low-temperature liquid. In order for cooling to occur using ambient air or room temperature water, which are easily accessible, the temperature at which the refrigerant condenses must be higher than those temperatures.
Stewed food in a pressure cooker will rise in temperature without boiling to about 120°C when pressure is applied. This makes it possible to cook at higher temperatures.
refrigerant Similarly, in the case of the "2." process, the condensation temperature can be raised by increasing the pressure. In the process of compression, low pressure and low temperature refrigerant are compressed in a compressor to increase pressure. At this time, heat of compression is also generated, so the temperature also rises, resulting in a high-temperature, high-pressure gas.
②Condensation
In the case of a room air conditioner, this corresponds to the outdoor unit. The high-temperature, high-pressure refrigerant in step ① is cooled and condensed using the surrounding air or water in the condenser. The temperature of the air or water used for cooling rises as the heat of condensation is released. In this way, the heat of evaporation absorbed when cooling the space and the heat of compression received from the compressor are released outside the cycle.
At this time, refrigerant changes from gas to liquid, but because it is changing state, its temperature does not change and it becomes a high-temperature, high-pressure liquid.
③ Expansion
The flow of high-temperature, high-pressure liquid is restricted by the expansion valve and then released, causing a sudden drop in pressure. At this time, some of refrigerant evaporates, and the heat of vaporization lowers the temperature of most of the remaining liquid. In this way, the liquid changes to a low-temperature, low-pressure liquid, which can then be easily evaporated in the evaporator.
④Evaporation
In the case of a room air conditioner, this corresponds to the indoor unit, and is the process that actually performs the cooling.
The low-temperature, low-pressure refrigerant evaporates in the evaporator by taking heat of evaporation from the surrounding air, which lowers the temperature of the surrounding air.
At this time, refrigerant changes state from liquid to gas, so there is no change in temperature and it becomes a low-temperature, low-pressure gas.
Table. Summary of refrigerant state during freezing cycle
| Four element parts | refrigerant condition | Temperature | Heat transfer | |
|---|---|---|---|---|
| ① Compression | Compressor | Low temperature/low pressure gas → high temperature/high pressure gas | Low temperature → High temperature | + Compression heat |
| ② Condensation | condenser | High-temperature, high-pressure gas → high-temperature, high-pressure liquid | High temperature (constant) | -Condensation heat (= -heat of evaporation - heat of compression) |
| ③ Expansion | Expansion valve | High temperature and high pressure liquid → Low temperature and low pressure liquid | High temperature → Low temperature | No heat balance |
| ④ Evaporation | evaporator | Low temperature/low pressure liquid → low temperature/low pressure gas | Low temperature (constant) | + Heat of evaporation |
Previous item: 1-3. Cooling principle
Next item: refrigerant
People who viewed this page also checked out these documents:
Inquiry
For product inquiries, quote requests, etc.
Please feel free to contact us.












